Respiratory compromise has been and remains a leading cause of death in people with spinal cord injury (SCI) in the United States and other high-income countries. Accordingly, in 2005 the United States Centers for Disease Control (CDC) elevated the priority of annual influenza (flu) vaccination for people with SCI. Despite this precedent–and that SARS-CoV-2 (“Coronavirus”) is primarily a respiratory virus—there have been no official (Government nor Institutional) recommendations for vaccination against COVID-19 in people with SCI.
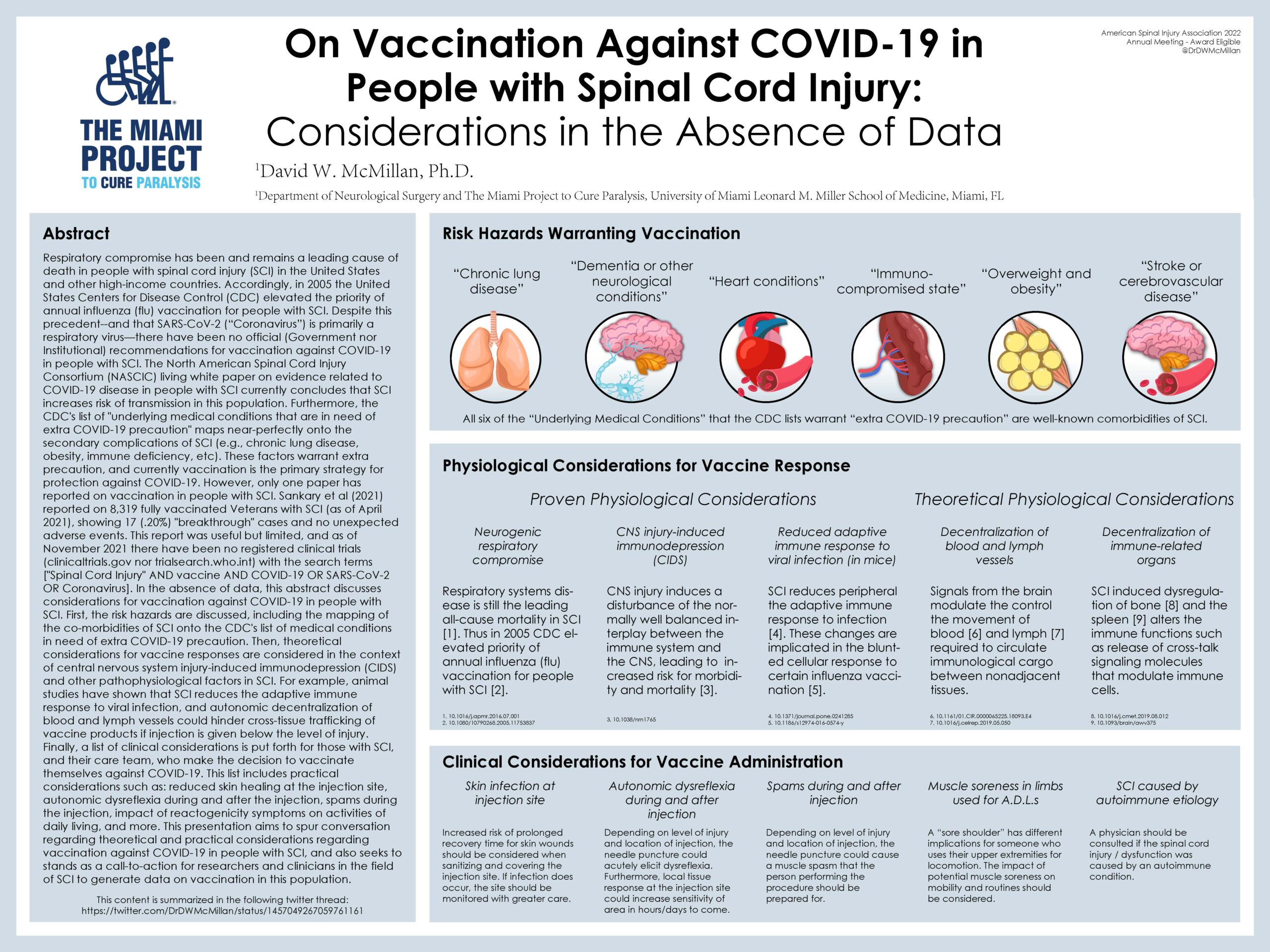
The North American Spinal Cord Injury Consortium (NASCIC) living white paper on evidence related to COVID-19 disease in people with SCI currently concludes that SCI increases risk of transmission in this population. Furthermore, the CDC’s list of “underlying medical conditions that are in need of extra COVID-19 precaution” maps near-perfectly onto the secondary complications of SCI (e.g., chronic lung disease, obesity, immune deficiency, etc). These factors warrant extra precaution, and currently vaccination is the primary strategy for protection against COVID-19. However, only one paper has reported on vaccination in people with SCI. Sankary et al (2021) reported on 8,319 fully vaccinated Veterans with SCI (as of April 2021), showing 17 (.20%) “breakthrough” cases and no unexpected adverse events. This report was useful but limited, and as of November 2021 there have been no registered clinical trials (clinicaltrials.gov nor trialsearch.who.int) with the search terms [“Spinal Cord Injury” AND vaccine AND COVID-19 OR SARS-CoV-2 OR Coronavirus]. In the absence of data, this abstract discusses considerations for vaccination against COVID-19 in people with SCI. First, the risk hazards are discussed, including the mapping of the co-morbidities of SCI onto the CDC’s list of medical conditions in need of extra COVID-19 precaution. Then, theoretical considerations for vaccine responses are considered in the context of central nervous system injury-induced immunodepression (CIDS) and other pathophysiological factors in SCI. For example, animal studies have shown that SCI reduces the adaptive immune response to viral infection, and autonomic decentralization of blood and lymph vessels could hinder cross-tissue trafficking of vaccine products if injection is given below the level of injury.
Finally, a list of clinical considerations is put forth for those with SCI, and their care team, who make the decision to vaccinate themselves against COVID-19. This list includes practical considerations such as: reduced skin healing at the injection site, autonomic dysreflexia during and after the injection, spams during the injection, impact of reactogenicity symptoms on activities of daily living, and more.
This presentation aims to spur conversation regarding theoretical and practical considerations regarding vaccination against COVID-19 in people with SCI, and also seeks to stands as a call-to-action for researchers and clinicians in the field of SCI to generate data on vaccination in this population.
