(November – 2019) Miami Project researchers are taking on spinal cord injury. After spinal cord injury (SCI), damage within the central nervous system triggers a natural cascade of events to protect surrounding tissue and repair the damaged spinal cord. The immune system mobilizes a sophisticated army of biological warfare specialists to the front lines of the SCI, where a variety of cell types contribute towards defensive and offensive efforts involved in the wound healing process.
White blood cells (monocytes) migrate toward the injury site and take on numerous responsibilities over the first few months. Early after injury, monocytes act as the clean-up crew, turning in to cells that surround and digest cellular debris and foreign substances (macrophages). This rapid-response team often must travel long distances to reach the battleground, with many originating from the spleen and, eventually, bone marrow. Macrophages persist within the central nervous system around the area of injury and continue to perform a variety of duties for many years. During that time, certain subtypes of macrophages seem to have beneficial effects and promote regeneration. Yet, other subtypes of macrophages appear to contribute to chronic inflammation, which limits the potential for neural repair and regeneration. While macrophages play a central role in the wound healing process after SCI, there are still a lot of unanswered questions.
To help clarify the role of macrophages after SCI, Dr. Jae Lee, Associate Professor at The Miami Project and Department of Neurological Surgery, recently published a review, entitled “The origin, fate, and contribution of macrophages to spinal cord injury pathology” in Acta Neuropathologica, along with Neuroscience program graduate students, Lindsay Milich and Christine Ryan. The Miami Project scientists describe the current state of knowledge regarding macrophages in the central nervous system following SCI and highlight potential strategies for reducing chronic inflammation after SCI.
“The fact that there are multiple sources of monocyte-derived macrophages at the injury site complicates the biology. However, the fact that these cells travel through the blood and have the innate ability to migrate to the injury site lend themselves as sort of a ‘Trojan horse’ for delivery of therapeutics for potential future interventions targeting injury,” said Dr. Lee.
Miami Project scientists are leading the way towards developing effective strategies for reducing inflammation and improving function after SCI. In a recent collaborative study with Dr. Nagi Ayad, Associate Professor of Psychiatry and Behavioral Sciences and Co-Director of the University of Miami Brain Tumor Initiative, Dr. Lee targeted a family of proteins (bromodomain and extraterminal domaincontaining proteins, BETs), which are important regulators of inflammation. Previous studies have shown that inhibition of BETs reduces inflammation in experimental models of arthritis, atherosclerosis, and inflammatory bowel disease.
To evaluate the effects on neuroinflammation, scientists injected a BET inhibitor (JQ1) into mice three hours after experimental SCI. By three days post-injury, markers of inflammation within the spinal cord were decreased and less immune cells were being recruited to the injury site. Despite this, the researchers did not observe improvements in locomotor function or reduction in spinal cord damage. Their findings were published in the journal of Experimental Neurology. Drs. Lee and Ayad suggest that different BET inhibitors with better drug-like properties or modes of drug delivery may ultimately decrease secondary injury and promote improved functional outcomes after SCI.
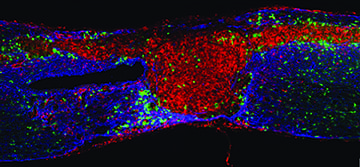
*photo: Macrophages and microglia are distributed around the spinal cord injury area. At 14 days after injury, microglia are located mostly around the astroglial scar area, and monocyte-derived macrophages are found within the lesion site, as well as in the astroglial scar.