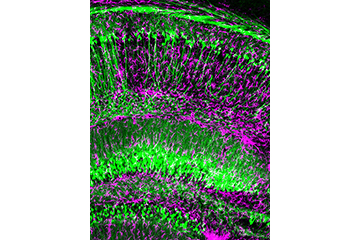
Dan Liebl, Ph.D. and Colleagues Awarded a 5-year $2.2 Million National Institute of Neurological Disorders and Stroke Grant Titled Stabilizing the tripartite synaptic complex following Traumatic Brain Injury
Daniel J. Liebl, Ph.D., Professor, Department of Neurological Surgery and The Miami Project to Cure Paralysis and colleagues recently received a 5 year $2.2 million National Institute of Neurological Disorders And Stroke (NINDS) Grant titled Stabilizing the tripartite synaptic complex following Traumatic Brain Injury. Synaptic damage is an attribute observed in all traumatic brain injuries (TBI) ranging from mild concussions to severe brain penetration, so identifying the mechanisms that induce synaptic damage is an important step in understanding how to protect the nervous system after injury.
Liebl and his team examine these injury mechanisms using highly innovative and novel approaches. Employing cell-specific, inducible, gene-targeted knockout mice, Liebl has shown that the phasic release of the co-transmitter D-serine from hippocampal glutaminergic neurons plays an important role in regulating synaptic function; however, following injury D-serine is suppressed in neurons but upregulated in astrocytes. Increased tonic release of astrocytic D-serine leads to sub-lethal synaptic damage over the first week post-injury. Identifying the conditions of these unique cellular sources and their differential functions has answered questions that have eluded researchers for over a decade, and led to a recent publication in the Journal of Clinical Investigation.
Liebl and colleagues went on to show that communication between astrocytes and synaptic membranes, in a structure called the tripartite synapse, and involves interactions between membrane proteins called ephrins and Eph receptors. In particular, synaptic signals activate EphB3 receptors in reactive astrocytes to regulate the excessive release of D-serine. Blocking EphB3 signaling or downstream mediators of D-serine production in the injured brain not only block D-serine synthesis but also led to enhanced synaptic stability, increase neuronal activity and improved behavioral recovery. These findings were published in Neurobiology of Disease.
Dr. Liebl believes, “In order to stabilize our brains after trauma, we will need to better understand the signaling mechanisms that regulated synaptic degeneration, and through this understanding we will be able to identify targets that can be developed for clinical applications.” Liebl and his team will continue to investigate the mechanisms that underlie synaptic damage, in part, by identifying the epigenetic modifiers that regulate important regulators of D-serine synthesis.
Traumatic brain injury is a devastating worldwide disorder, and is believed to become the third most prevalent health concern contributing to patient mortality by 2020. The annual cost of TBI is estimated at ~$80 billion, so developing therapeutic treatments that can protect patients ranging from repetitive concussions to severe injuries is important.
This research was supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS098740.